THE INFLUENCE OF SOME FACTORS ON THE EXTRACTION OF URANIUM AND THORIUM FROM WATER SOLUTIONS BY CHLORATED NAPHTENIC ACIDS
Abstract
The paper present the results of the investigation of the extraction of micro-concentrations of uranium and thorium by naphthenic acids (NA) and chlorinated naphthenic acids (CNA) from the water solutions of corresponding salts, as well as by chlorinated naphthenic acids in the presence of chlorides and sulphates of alkali metals.
Based on the results of the extraction of thorium and uranyl ions (0.5 M and 1 M, accordingly) by the solutions of NA and CNA it is shown, that the extraction of thorium ions (0.5 M) and uranyl ions (1 M) by CNA solutions starts at pH = 1.5 and pH = 1.8 accordingly. At pH = 2.76 and pH = 4.6 they completely transfer into the organic phase. At the same conditions the extraction of thorium by NA starts only at pH=2.36, and for uranium─ just at pH = 5.2. It must be mentioned, that under the same conditions, the pH of these ion extractions shifts approximately in one unit to the right. Further increasing the pH of the water solution, both for thorium ions and for uranium ions, leads to a step-by-step lowering of coefficient distribution and an emulsion appearing on the boundary of phases, as a result of which their stratification becomes difficult.
The phases shift, in case of thorium extraction, takes place at pH > 4, and in the case of uranium extraction at pH >6. The emulsion is easily destroyed when adding the minimum quantities of high alcohols into the organic phase.
It has been shown, that extraction recovery of microquantities of uranium and thorium out of water solutions by chlorinated naphthenic acids depends on the pH of solution and addition of the salts of alkali metals.
It has been found, that the presence of NaCl in the solution and the rise of its concentration (from 0.25 N to 2 N) leads to the shift of pH medium of optimum extraction to acidic field, starting from pH = 4.5 to pH = 3 for uranyl-ions, and for thorium-ions from pH = 3.27 to pH = 2.75.
In case of containing Na2SO4 in the solution, maximum extraction of thorium and uranium is marked by the shift of pH medium in the direction from pH = 4 to pH = 4.65 and pH = 5.6 respectively, further rise the pH of solution leads to a the shift of phases.
Thus, under equal conditions the pH of equilibrium of a water solution in which 50% of thorium and uranyl ions are extracted correspondingly results in:
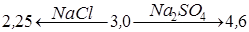 and
and 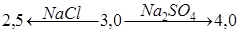
The distribution of CAN between the different organic solvents and water the presence of a dimer form of these acids in the inert diluents has been discovered.
It should be mentioned that the nature of the investigated solvents (kerosene, benzene, chloroform and carbon tetrachloride) doesn't influence the degree of the extraction of the studied ions.
In accordance with this, the extraction reaction of thorium and uranyl-ions with CNA can be written as follows:
Th4++4/2(XHK)=ThR4−2HR+4H+;UO2+2=4/2[(XHK)2]↔UO2R2⋅2(XHK)4+2H+Th4++4/2(XHK)=ThR4−2HR+4H+;UO22+=4/2[(XHK)2]↔UO2R2⋅2(XHK)4+2H+The optimum ratios CNA: Th4+=3.4Th4+=3.4 and CNA: UO2+2>6UO22+>6 have been discovered, that is equal to maximum extraction of presented ions by chlorate naphthenic acids.
Downloads
References
2. Turanov A. N., Karandashev V. K., Sharova E. V., Artyushin O. I., Odinets I. L. Radiochemistry, 2010, vol. 52, no. 3, pp. 258-263. DOI: 10.1134/S1066362210030069
3. Seydel D. K. IAEA Bulletin, 1980, vol. 23, no. 2, p. 29-31.
4. Yakshin V.V., Tsarenko N. A., Koscheev A. M., Tsivadze A.Y. Russ. J. Inorg. Chem., 2011, vol. 56, no. 12, pp. 1997-2000. DOI: 10.1134/S0036023611120473
5. Volk V. I., Vahrushin A. Yu., Mamaev S. L. Radiochemistry, 1999, vol. 41, no. 3. pp. 120-123.
6. Mayorov V. G., Nikolayev A. I. Russ. J. Appl. Chem., 2006, vol. 79, no. 7, pp. 1196-1199. DOI: 10.1134/S1070427206070305
7. Turanov A. N., Karandashev V. K., Yarkevich N. N., Safronova N. I., Rodigina N. I., Fedoseev A. M. Radiochemistry, 2007, vol. 49, no. 6, pp. 618-623. DOI: 10.1134/S1066362207060148














